In 2004 a group of scientists lead by May-Britt and Edvard Moser discovered neurons in the entorhinal cortex of rats that exhibit a peculiar form of spatially correlated activity [1,2]. The entorhinal cortex is part of the hippocampal-parahippocampal region of the mammalian brain and the region is known to play an important role for episodic memory as well as orientation and navigation. It hosts a number of cells that exhibit spatially correlated activity [3,4,1,2,5]. A prominent example for such cells are hippocampal place cells that were discovered in the 1970s by O’Keefe and Dostrovsky [6,7]. Place cells are neurons that fire (i.e., become active) when the animal is at specific locations in its environment. Each place cell encodes one particular location: the cell’s firing field. The cells found by May-Britt and Edvard Moser, termed grid cells, behave in a similar fashion as place cells. However, they possess not just one but multiple, discrete firing fields that are arranged in a regular, hexagonal grid that spans the entire environment (Fig. 1).
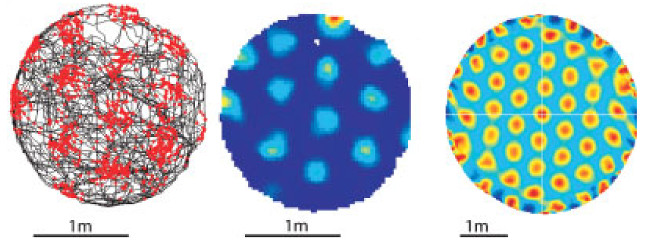
Located just one synapse upstream of the hippocampus, grid cells are assumed to be an important source of spatial information to place cells [9,10]. In particular, grid cells are generally considered to be a central part of a path integration system that provides some kind of metric for space [8]. As Burgess [11] points out: “There has been a surprising rapid and general agreement that the computational problem to which grid cells provide a solution is “path integration” within an allocentric reference frame." This consensus is reflected by the fact that the vast majority of computational models of grid cells proposed so far incorporate mechanisms of path integration as integral parts to explain the hexagonal firing patterns of grid cells. Although existing computational models cover a wide range of possible mechanisms and focus on different aspects of grid cell activity [8,12,13,14,15,16], the models share the common approach of explaining grid cells and their behavior as specialized, functional components within a system dedicated for orientation and navigation.
This hypothesis is supported by early experimental results that highlight a number of interesting grid cell properties [17]. Grid cells in the medial entorhinal cortex are topographically organized in groups. Neighboring grid cells within a group exhibit similar grid spacing, field size and grid orientation. Starting from the postrhinal border grid spacing and field size increase along the dorsoventral axis of the medial entorhinal cortex [2,18,19]. The locations of the individual firing fields of a grid cell are anchored to external, visual landmarks in the environment, though the locations remain stable if those landmarks are temporarily not visible, e.g., in darkness [2]. Further experimental results show that the firing fields of grid cells follow rapid changes of an environment’s geometry and realign if the environment changes entirely [20,21,22]. Yet, during all these changes the firing field locations of a grid cell remain stable relative to the fields of neighboring grid cells. This relative stability is one of the main characteristics that support the navigation system hypothesis. For an extensive survey of grid cell properties see [17].
In more recent years new experimental results have emerged that weaken the idea that grid cells are a specialized component in a system for orientation and navigation. It seems that the activity of grid cells is less absolute and more context dependend than previously thought [23,24]. Moreover, grid-cell-like activity could be observed in other parts of the mammalian brain including the pre- and parasubiculum in rats [25] as well as the hippocampus, parahippocampal gyrus, amygdala, cingulate cortex, and frontal cortex in humans [26]. In addition, grid activity was witnessed in cells that do not encode locations in space but rather, e.g., viewing directions [27], auditory frequencies [28], or even abstract concepts [29]. Together, these new results suggest that grid-cell-like activity might be a more widespread phenomenon within the mammalian brain. Based on this premise I was able to find a computational model of grid cells that does not rely on assumptions that require the presence of a presumed navigational system. The details of this model are described in section grid cell model. In particular, the model is capable of describing new phenomena observed in recent experiments like the grid-like activity of neurons in response to eye movements (see section encoding of visual space). It also sheds some new light on the possible operation of the hippocampal circuit as it might relocate the locus of a generally assumed occurrence of pattern separation from the dentate gyrus to the entorhinal cortex.
References
1
,
Spatial Representation in the Entorhinal Cortex,
In: Science, volume 305, pages 1258-1264, number 5688, 2004,
[doi]
2
,
Microstructure of a spatial map in the entorhinal cortex,
In: Nature, volume 436, pages 801-806, number 7052, 2005,
[doi]
3
,
Head-direction cells recorded from the postsubiculum in freely moving rats. I. description and quantitative analysis,
In: The Journal of Neuroscience 10(2), 420–435, 1990,
[doi]
4
,
Spatial selectivity of unit activity in the hippocampal granular layer,
In: Hippocampus 3(2), 165–182, 1993,
[doi]
5
,
Representation of geometric borders in the entorhinal cortex,
In: Science volume 322, pages 1865–1868, number 5909, 2008,
[doi]
6
,
The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat,
In: Brain Research 34(1), 171 – 175, 1971,
[doi]
7
,
Place units in the hippocampus of the freely moving rat,
In: Experimental Neurology 51(1), 78 – 109, 1976,
[doi]
8
,
A metric for space,
In: Hippocampus 18(12), 1142–1156, 2008,
[doi]
9
,
From grid cells to place cells: A mathematical model,
In: Hippocampus 16(12), 1026–1031, 2006,
[doi]
10
,
Entorhinal cortex grid cells can map to hippocampal place cells by competitive learning,
In: Network: Computation in Neural Systems 17(4), 447–465, 2006,
[doi]
11
,
Grid cells and theta as oscillatory interference: Theory and predictions,
In: Hippocampus 18(12), 1157–1174, 2008,
[doi]
12
,
Grid cells: The position code, neural network models of activity, and the problem of learning,
In: Hippocampus 18(12), 1283–1300, 2008,
[doi]
13
,
Computational models of grid cells,
In: Neuron 71(4), 589 – 603, 2011,
[doi]
14
,
Neural mechanisms of self-location,
In: Current Biology 24(8), R330 – R339, 2014,
[doi]
15
,
Spatial coding and attractor dynamics of grid cells in the entorhinal cortex,
In: Current Opinion in Neurobiology 25(0), 169 – 175, 2014,
[doi]
16
,
Network mechanisms of grid cells,
In: Philosophical Transactions of the Royal Society B: Biological Sciences 369(1635), 2014,
[doi]
17
,
A Survey of Entorhinal Grid Cell Properties,
In: arXiv e-prints, arXiv:1810.07429 (Oct. 2018), arXiv:1810.07429. arXiv: 1810.07429 [q-bio.NC], 2018,
[pdf|bibtex]
18
,
Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex,
In: Hippocampus, 18(12):1200–1212, 2008,
[doi]
19
,
The entorhinal grid map is discretized,
In: Nature, 492(7427):72–78, 2012,
[doi]
20
,
Hippocampal remapping and grid realignment in entorhinal cortex,
In: Nature, 446(7132):190–194, 2007,
[doi]
21
,
Grid cell firing patterns signal environmental novelty by expansion,
In: Proceedings of the National Academy of Sciences, 109(43):17687–17692, 2012,
[doi]
22
,
Experience-dependent rescaling of entorhinal grids,
In: Nat Neurosci, 10(6):682–684, 2007,
[doi]
23
,
Visual landmarks sharpen grid cell metric and confer context specificity to neurons of the medial entorhinal cortex,
In: eLife, 5:e16937, 2016,
[doi]
24
,
Context-dependent spatially periodic activity in the human entorhinal cortex,
In: Proceedings of the National Academy of Sciences, 2017,
[doi]
25
,
Grid cells in pre- and parasubiculum,
In: Nat Neurosci, 13(8):987–994, 2010,
[doi]
26
,
Direct recordings of grid-like neuronal activity in human spatial navigation,
In: Nat Neurosci, 16(9):1188–1190, 2013,
[doi]
27
,
A map of visual space in the primate entorhinal cortex,
In: Nature, 491(7426):761–764, 11, 2012,
[doi]
28
,
Mapping of a non-spatial dimension by the hippocampal & entorhinal circuit,
In: Nature, 543(7647):719–722, 2017,
[doi]
29
,
Organizing conceptual knowledge in humans with a grid-like code,
In: Science (New York, N.Y.), 352(6292):1464–1468, 2016,
[doi]


